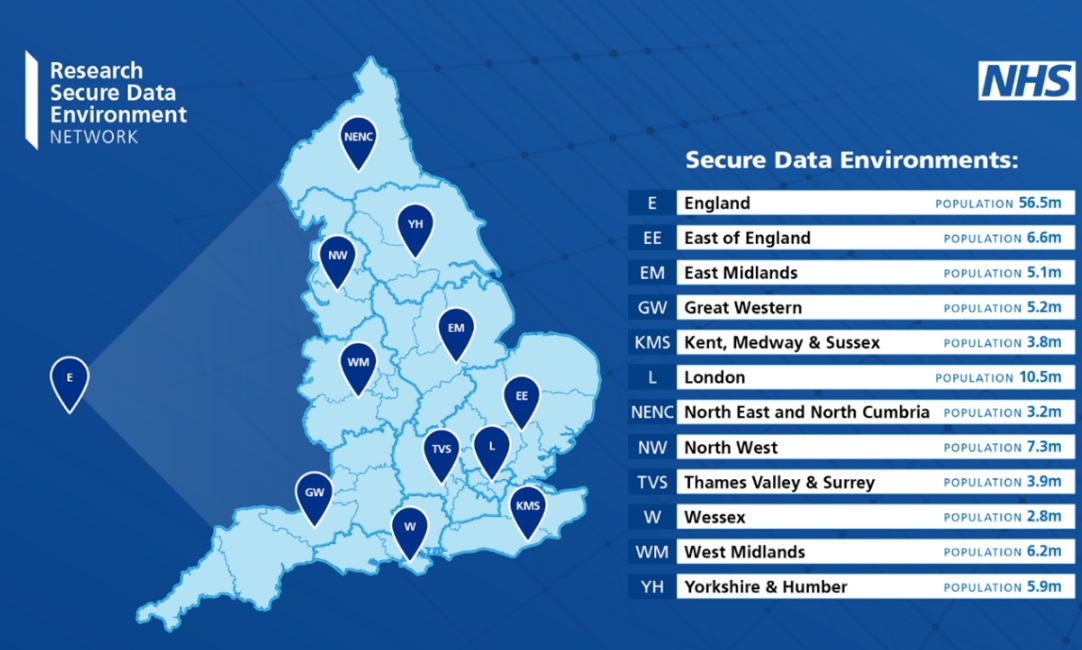
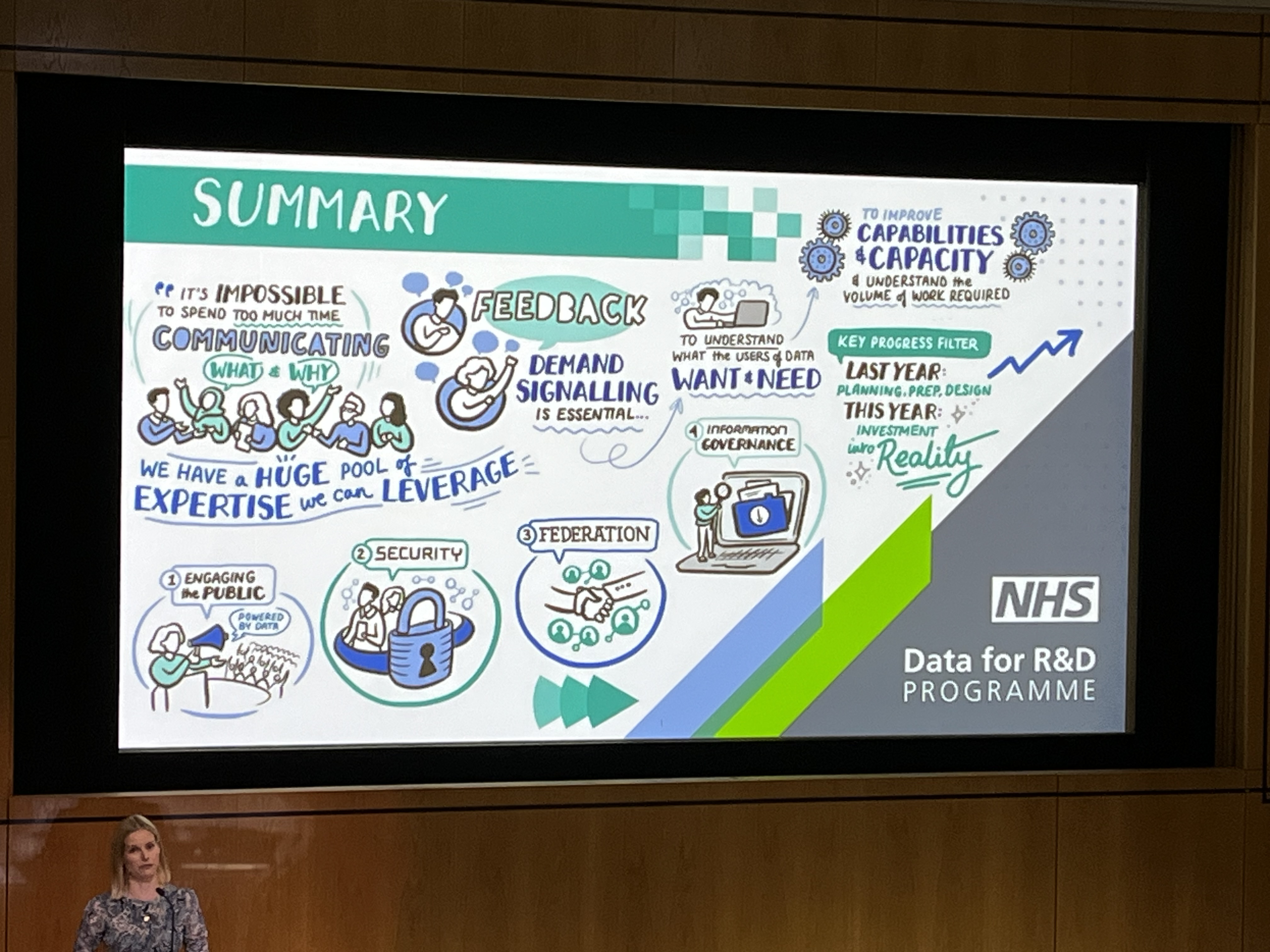
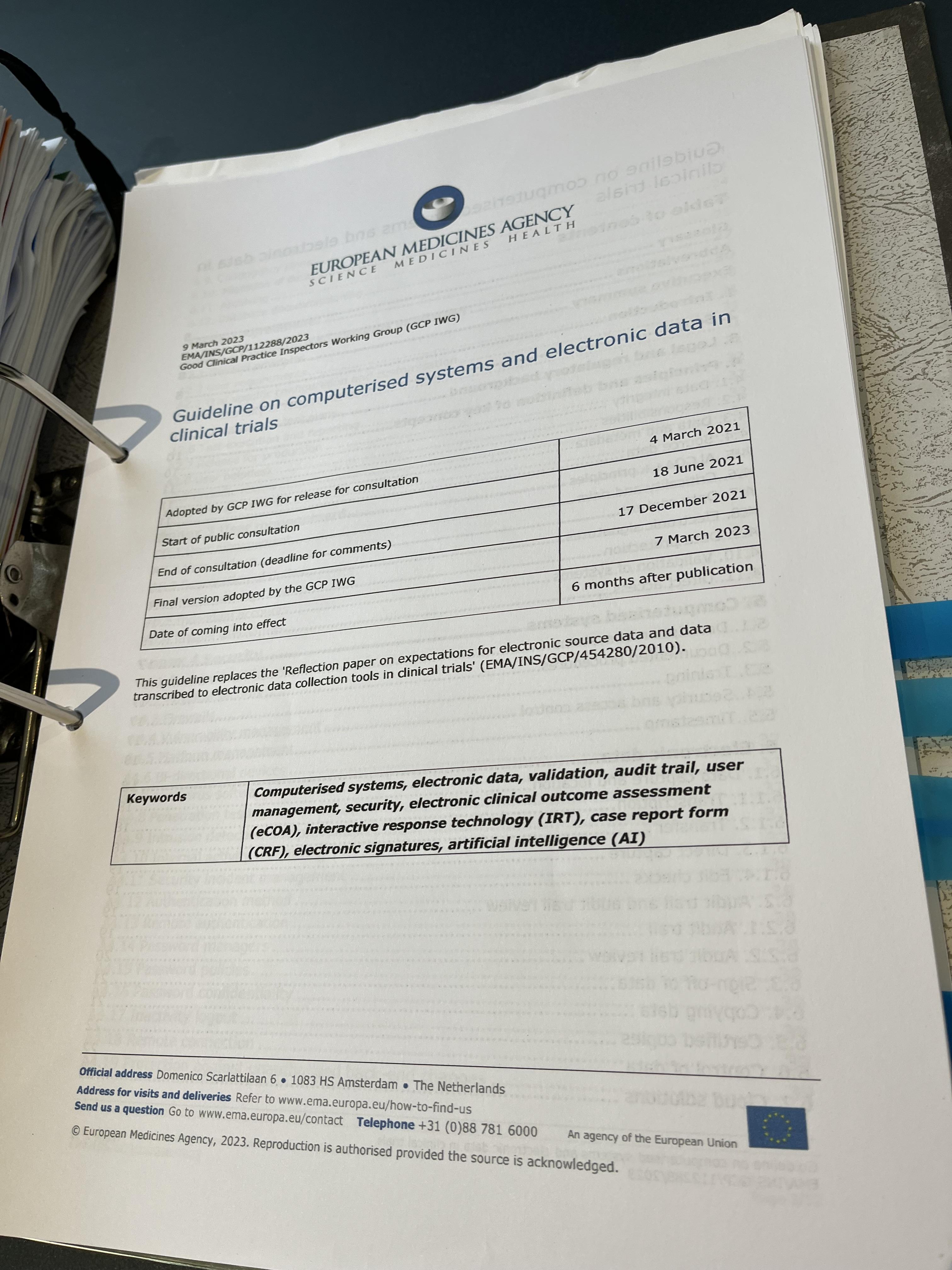
RISG Consulting provides professional services to academic and NHS clinical research organisations including strategic IS/IG advice, staffing and infrastructure, regulatory expectations for clinical trial IT systems (system validation, data integrity, regulatory audit preparation), NHS Data Security and Protection Toolkit submissions, specification and procurement of EDC systems and all aspects of information system governance.
RISG Consulting has worked widely with UK academic and NHS clinical trials units, NIHR and pharma.